How to create deuterium oxide
چهارشنبه, ۱۵ آذر ۱۳۹۶، ۱۲:۲۰ ب.ظ
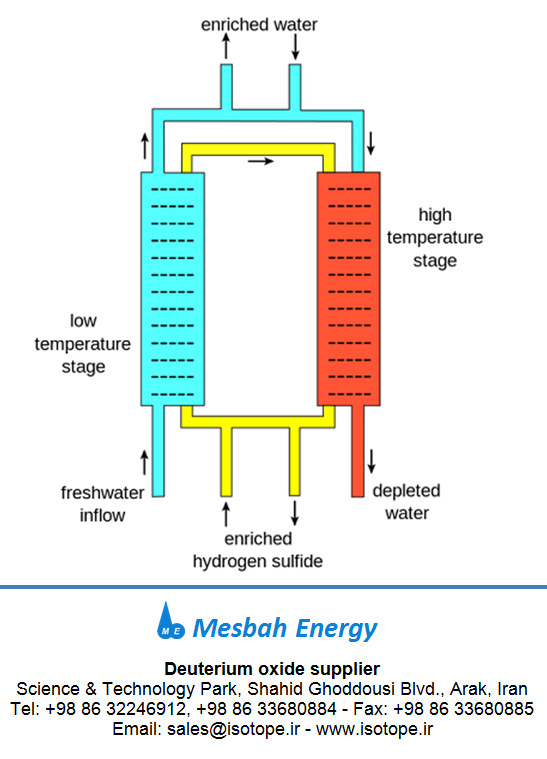
Fig 1.Production of deuterium oxide (heavy water) - Girdler sulfide process
As semi heavy water, HDO occurs naturally on
Earth in regular water at a proportion of 1 part per 3200, it may be separated
from regular water by distillation or electrolysis and also by various chemical
exchange processes, all of which exploit a kinetic isotope effect. In short,
the difference in mass between the two hydrogen isotopes translates into a
difference in the zero-point energy and thus into a slight difference in the
speed at which the reaction proceeds. Once HDO becomes a significant fraction
of the water, deuterium oxide (heavy water) will become more prevalent as well
as water molecules trade hydrogen atoms very frequently. To produce pure
deuterium oxide by distillation or electrolysis requires a large cascade of
stills or electrolysis chambers, and consumes large amounts of power, so the
chemical methods are generally preferred. The most important chemical method is
the Girdler Sulfide process.
Source:
https://en.wikipedia.org/wiki/Heavy_water#Production